Less Paperwork, More Progress
The CTMS for Smarter, Faster Trials
![]() About
About
eVision CTMS
Simplify. Optimize. Accelerate.
eVision CTMS is a modern, intuitive, and efficient clinical trial management system designed for today’s fast-paced research environment. Whether you are a Sponsor or CRO eVision streamlines trial operations and enhances resource allocation—without the complexity of traditional CTMS platforms.
Effortless Study Oversight
A user-friendly, web-based platform that streamlines trial management without unnecessary complexity.
Smarter Site & Resource Management
Track site visits, investigator performance, and CRA workload in one centralized system.
Why eVision
Automate every phase of your study.
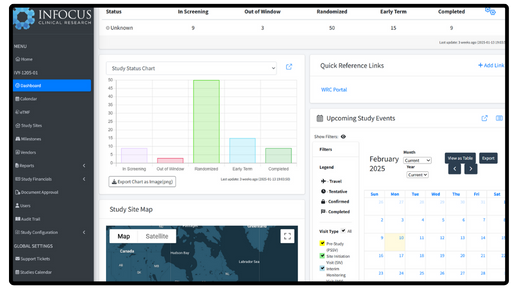
Integrated Study Calendar
Travel, visits, and document management seamlessly combined for optimized scheduling.
Real-Time Insights & Compliance
Actionable dashboards keep studies on track while ensuring secure, 21 CFR Part 11-compliant data management.
![]() Features
Features






![]() eTMF
eTMF
Managing trials AND keeping your docs in order?
Yep, we do both
eVision CTMS includes a robust electronic Trial Master File (eTMF) that ensures sponsors and CROs maintain compliance, organization, and efficiency in clinical trial documentation. Designed for regulatory adherence, eVision eTMF simplifies document collection, version control, and audit readiness.
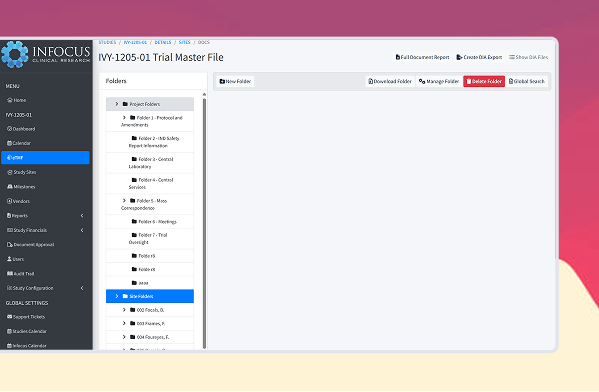
Why our eTMF over the others?
Because we’re better
Regulatory Compliance & Security
Centralized Document Management
Automated Workflows & Notifications
Seamless Integration with CTMS Functions
Efficient Search & Retrieval
Sponsor & Regulatory Submission Readiness
![]() Features
Features






![]() Customers
Customers
Powering clinical trials with a CTMS built for you.
Trusted by leading research teams and top pharmaceutical organizations

![]() Testimonials
Testimonials
“Porttitor phasellus est vivamus porta ut blandit. Quis eget lobortis nullam fringilla torquent dis dui.”
Bryce Wells
Vice President at InFocus Clinical Research
Take Control of Your Clinical Trials with eVision CTMS
Choose the perfect plan for your research needs and keep your trials running smoothly. Effortless compliance, real-time insights, and seamless collaboration—so you can focus on what matters most.
Essentials
- Up to 3 Trials
- Up to 10 seat licenses
- Up to 10 seat licenses
- Max 1GB File Storage
- Business Hours Support
- Core Reports and Trackers
- TMF Document Catalogue
Pro
- Up to 10 Trials
- Up to 20 seat licenses
- Max 5GB File Storage
- Pro Support
- Customized Document
- Catalog
- Custom Reports
Enterprise
- White label cloud hosted instance
- Integrations with popular EDC platforms
- API Access
- Custom, purpose-built modules
Transforming Clinical Trials for a Smarter Future.
Connect with us
Have questions or want to streamline your clinical trials?

